VTX-31
The global prevalence of Type 2 diabetes is estimated at 300 million patients, accounting for 12% of global healthcare spending. In the United States, 38 million Americans suffer from diabetes representing 12% of the total population. Left untreated, diabetes leads to the development of kidney disease, neuropathy,
retinopathy, poor wound healing, GI dysfunction,
cognitive impairment, liver fibrosis and
cardiovascular disease. The total healthcare
costs for managing diabetes to the US
healthcare system is $413 billion. Glucose
lowering drugs account for 12% of total diabetes
care at $48 billion. Sales of the Top 5 agents for
2023 totaled $33 billion.

Even at a 15% effect for an agent that
decreases disease progression, the downstream
savings to the healthcare system would amount
to at least $55 billion.
The Need: Current Type 2 Therapies Lack Durable Glucose Control
The GRADE trial sponsored by the NIH NIDDK Institute evaluated agents from all classes for long-term glucose control over a 4 year period. Patients taking metformin were enrolled and study drugs were added to their treatment regimen. At the conclusion of the study, 71% of the patients did not maintain their target HbA1c, the gold standard measure for glucose control, due to ongoing disease progression.
While developed to exert glucose lowering effects, none of the current therapies were designed to directly impact the underlying disease pathophysiology

4-HNE Induces Lipotoxicity And Insulin Resistance
With overnutrition, high levels of free fatty acids (FFA) are made from glucose and stored as fat in adipose cells. FFAs are also metabolized in the mitochondria through the oxidation cycle. During this process, highly reactive lipoperoxide byproducts are produced, including 4-HNE (4-hydroxynoneal). Under normal circumstances, 4-HNE is deactivated by cellular anti-oxidants such as glutathione. However, in obesity and Type 2 diabetes, larger amounts of 4-HNE are produced due to the increased levels of FFAs that overwhelms the anti-oxidant system. As a result, 4-HNE can bind irreversibly to proteins giving rise to adducts and impair their normal metabolic functioning. The GLUT4 transporter that shuttles glucose from the blood into muscle and fat cells is one of the proteins inactivated by 4-HNE which causes insulin resistance. As a consequence, blood levels of glucose are elevated and adipose cells release stored fatty acids due to the loss of feedback control of metabolic processes.

Other tissues and proteins that are inactivated by lipotoxicity include:
-
Pancreatic calmodulin: impaired insulin release and production, beta-cell death
-
Liver ATPase: loss of mitochondrial integrity, decreased ATP synthesis, fatty liver
-
Glutathione synthetase; decreased glutathione synthesis, loss of anti-oxidant
VTX-31 Directly Addresses Underlying Cause Of Insulin-Resistance
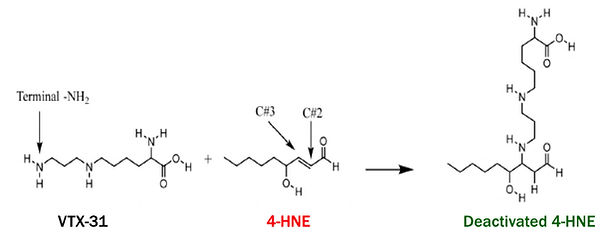
-
Orally-available compound specifically designed to bind and neutralize 4-HNE
-
Prevents lipotoxicity by decreasing its availability
-
Decreases protein adduct formation in adipose, pancreas and liver
-
Restores GLUT4 glucose uptake in brown adipose tissue and muscle
-
Decreases HbA1c levels in genetic and diet-induced animal models of disease

VTX-31 Exhibits Excellent Drug Properties
-
Roughly 95% oral bioavailability
-
No significant liver metabolism; 99% excretion by kidneys as unchanged compound
-
Half-life supports 2x/day dosing at estimated single doses around 300 mgs
-
Preliminary acute toxicity studies in rat, dog and monkey indicate VTX-31 is well-tolerated with no major adverse events seen at therapeutic doses
-
6-step simple, low cost synthesis; 1-year minimum stability in capsule form

